Proper Handling and Maintenance Practices with Endoscope Storage Cabinet Guidelines
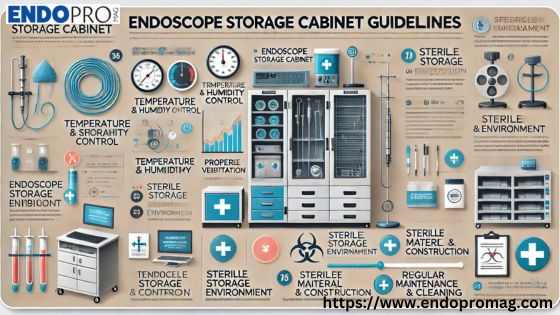
Maintaining a sterile environment is critical in modern healthcare settings, particularly in endoscopy units where reusable medical devices like endoscopes must be stored safely. To avoid cross-contamination and preserve the integrity of medical instruments, healthcare professionals must adhere to Endoscope Storage Cabinet Guidelines. These guidelines not only enhance patient safety but also ensure compliance with international healthcare standards. At Endopromag, we understand the importance of proper endoscope storage and offer expert-designed solutions and tips to help healthcare facilities meet and exceed industry requirements.
The Importance of Following Endoscope Storage Cabinet Guidelines
Improper storage of endoscopes after reprocessing can lead to serious health risks, including microbial contamination. The Endoscope Storage Cabinet Guidelines are designed to prevent these risks by providing specific protocols for drying, storing, and handling endoscopes post-cleaning. Implementing these best practices reduces infections, prolongs equipment life, and meets regulatory expectations.
Hospitals and clinics are required to maintain an environment that prevents recontamination of cleaned endoscopes. Following Endoscope Storage Cabinet Guidelines helps facilities meet infection prevention protocols laid out by regulatory authorities such as CDC, AORN, and SGNA.
Key Features of an Ideal Endoscope Storage Cabinet by Endopromag
At Endopromag, our storage cabinets are manufactured to comply with the latest Endoscope Storage Cabinet Guidelines. Below are the features every compliant cabinet should have:
-
Ventilation System: A high-efficiency particulate air (HEPA) filtered airflow system helps dry the internal channels of endoscopes and maintain a clean air environment inside the cabinet.
-
Vertical Hanging Design: The guidelines recommend hanging endoscopes vertically to avoid bends and allow proper drying of internal lumens.
-
Drip Trays: Removable trays should be placed under each endoscope to catch any residual fluid, reducing the risk of microbial growth.
-
Secure and Traceable Storage: Lockable doors and barcode tracking systems ensure that only trained personnel have access to the endoscopes and that all storage activities are logged and monitored.
These features directly align with Endoscope Storage Cabinet Guidelines and help healthcare facilities maintain consistent cleanliness and instrument readiness.
Proper Drying as a Core Requirement in Endoscope Storage Cabinet Guidelines
One of the most critical aspects of the Endoscope Storage Cabinet Guidelines is ensuring that the endoscope is completely dry before storage. Moisture inside the scope can lead to microbial proliferation. Therefore, cabinets must facilitate both external and internal drying.
Endopromag cabinets are equipped with channel-purging systems that flush air through the internal lumens, reducing drying time and ensuring compliance. This built-in mechanism helps prevent the formation of biofilms and supports long-term patient safety.
Storage Time Limits According to Endoscope Storage Cabinet Guidelines
The Endoscope Storage Cabinet Guidelines often recommend specific storage duration limits to maintain hygiene. The typical storage time without needing reprocessing again ranges from 72 hours to 7 days, depending on the cabinet’s design and environmental conditions.
Endopromag ensures our cabinets are tested to support these durations by maintaining HEPA-filtered environments and keeping scopes isolated from contaminants. Automatic door locks, internal environment monitoring, and alarm alerts are incorporated to reinforce adherence to these standards.
Routine Cleaning and Maintenance of Cabinets
Cleanliness of the cabinet itself is a crucial part of the Endoscope Storage Cabinet Guidelines. Endoscope storage units must be routinely wiped with hospital-grade disinfectants to avoid any indirect contamination.
At Endopromag, we offer training modules along with our storage systems to guide staff through cabinet maintenance procedures. Built with antimicrobial-coated surfaces and easy-to-clean materials, our cabinets are designed for healthcare environments with strict hygiene requirements.
Staff Training for Better Compliance
Even the best storage cabinet won’t help if the staff is not trained to follow the Endoscope Storage Cabinet Guidelines. All team members involved in endoscope handling should be trained to:
-
Properly dry and hang endoscopes.
-
Understand time limits and signs of contamination.
-
Clean the cabinet regularly and track each scope’s usage history.
Endopromag provides documentation, tutorial videos, and on-site training as part of our installation package, ensuring every facility that installs our cabinets is set up for success.
Addressing Common Errors in Endoscope Storage
Many healthcare institutions unknowingly violate Endoscope Storage Cabinet Guidelines by making small but impactful errors:
-
Leaving moisture in lumens: Leads to microbial contamination.
-
Improper cabinet airflow: Causes insufficient drying.
-
Overcrowding cabinets: Prevents proper ventilation.
Our experts at Endopromag help facilities assess their current setups and recommend improvements based on audits that compare current practices with updated Endoscope Storage Cabinet Guidelines.
Smart Storage Solutions from Endopromag
With technology transforming healthcare, smart storage is now an integral part of compliance. Endopromag has integrated smart sensors and IoT capabilities in its storage cabinets to improve adherence to Endoscope Storage Cabinet Guidelines. These include:
-
Real-time monitoring of internal temperature and humidity.
-
Alerts for overdue scope retrieval.
-
Digital logs to track usage, drying duration, and access control.
These advanced features enable healthcare institutions to maintain traceability, accountability, and a high standard of patient care.
Regulatory Compliance and Accreditation
Following Endoscope Storage Cabinet Guidelines also helps institutions earn or maintain accreditation from bodies like The Joint Commission (TJC) and Centers for Medicare and Medicaid Services (CMS). These organizations look for evidence of best practices in infection control, and storage compliance plays a big part.
Facilities using Endopromag storage systems find it easier to pass inspections and audits because our cabinets are pre-certified and designed to meet or exceed regulatory benchmarks.
About Endoscope Storage Cabinet Guidelines
1. What is the purpose of Endoscope Storage Cabinet Guidelines?
These guidelines are designed to prevent contamination and extend endoscope usability through proper drying, storing, and handling procedures.
2. How long can endoscopes be stored without reprocessing?
Most guidelines allow for storage durations between 72 hours and 7 days if stored in a compliant cabinet like those from Endopromag.
3. What makes Endopromag cabinets guideline-compliant?
HEPA filters, vertical hanging, channel-drying features, and secure tracking systems make our cabinets fully compliant.
4. Why is drying important in endoscope storage?
Moisture leads to microbial growth. Proper drying—both internal and external—is essential to prevent infections.
5. Do these guidelines help with regulatory audits?
Yes, adhering to Endoscope Storage Cabinet Guidelines ensures compliance with regulatory bodies like TJC and CMS.
Choosing the right cabinet can make a difference in how well a facility complies with Endoscope Storage Cabinet Guidelines. At Endopromag, we’ve engineered our systems with every important guideline in mind—from proper drying technology and airflow to smart tracking and ease of use.
When hospitals, surgery centers, and clinics partner with Endopromag, they benefit from:
-
State-of-the-art, guideline-compliant storage systems.
-
Enhanced safety for patients and healthcare staff.
-
Long-term durability and return on investment.
-
End-to-end training and support for implementation.
By aligning with Endopromag and implementing approved Endoscope Storage Cabinet Guidelines, facilities can safeguard their operations while maintaining top-tier clinical standards.






Leave a Comment